Have a question for us? Please reach out and we will respond within 24-48 hours.
support@reframebeauty.com
Participant
/
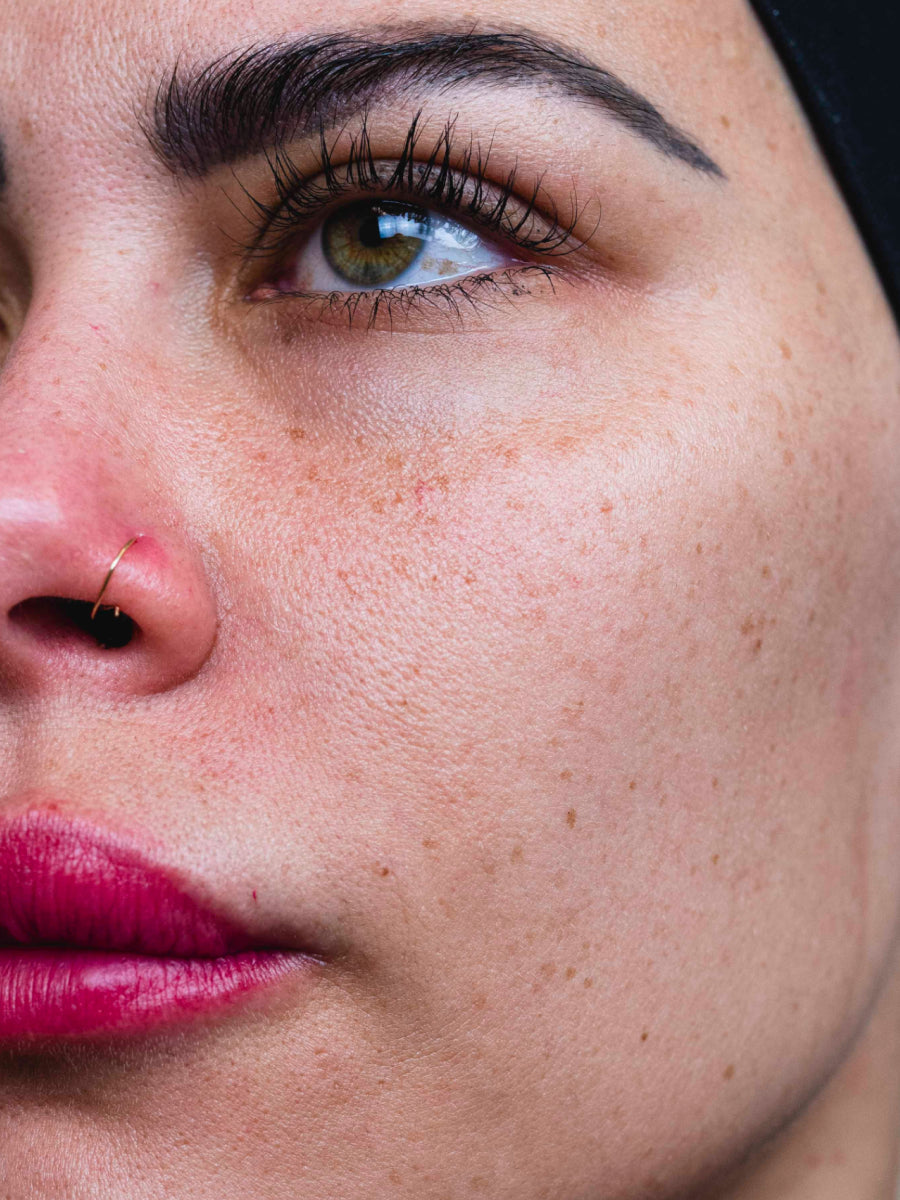
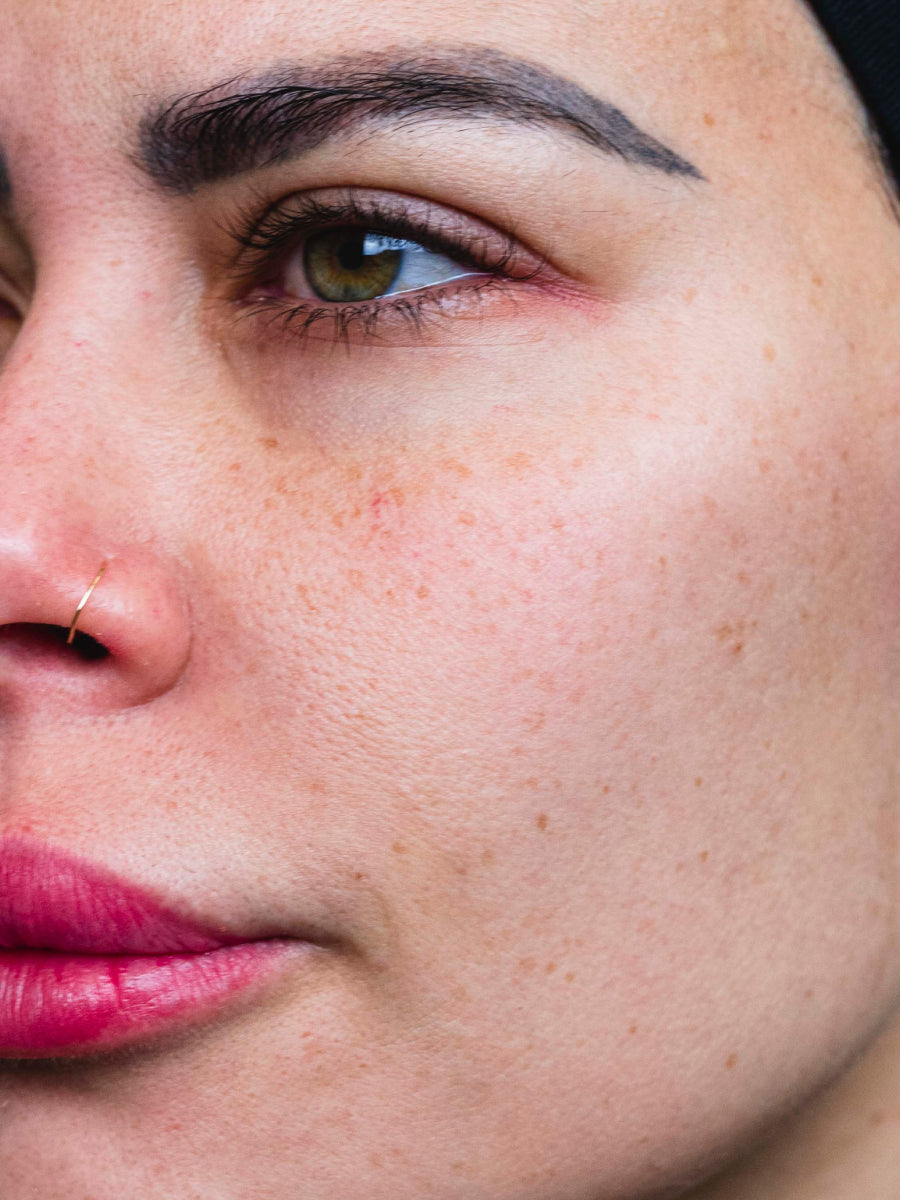
- Age: 36
- Fitzpatrick Scale: 3
- Treatment Duration: 4 weeks
- Featured Products: Circadian Cream
Featured in this trial

Circadian Cream
Overnight Collagen Seal
$135

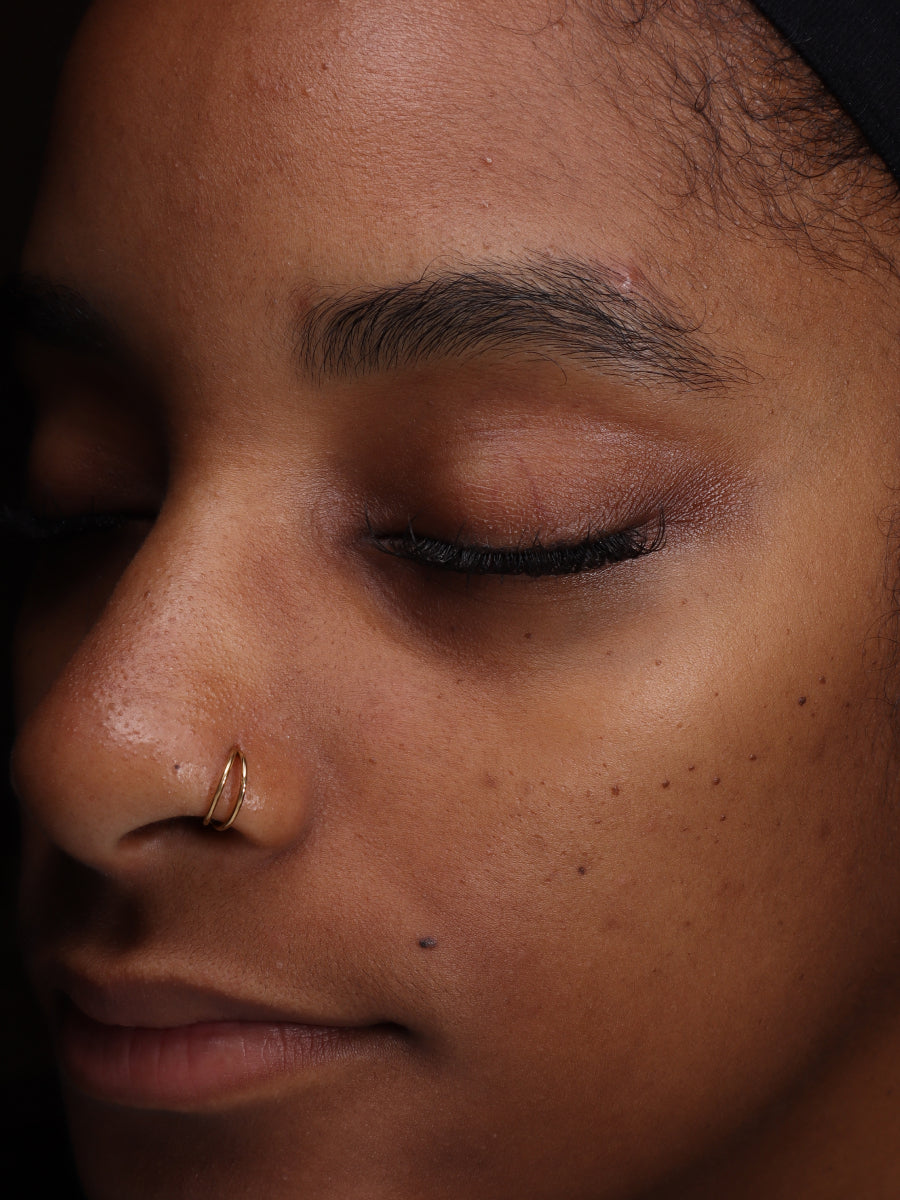
- Age: 26
- Fitzpatrick Scale: 5
- Treatment Duration: 8 weeks
- Featured Products: Circadian Cream
Featured in this trial

Circadian Cream
Overnight Collagen Seal
$135


- Age: 40
- Fitzpatrick Scale: 5
- Treatment Duration: 8 weeks
- Featured Products: Circadian Cream
Featured in this trial

Circadian Cream
Overnight Collagen Seal
$135


- Age: 30
- Fitzpatrick Scale: 3
- Treatment Duration: 8 weeks
- Featured Products: Pigment Processor
Featured in this trial

Pigment Processor
Daily Brightening Serum
$115
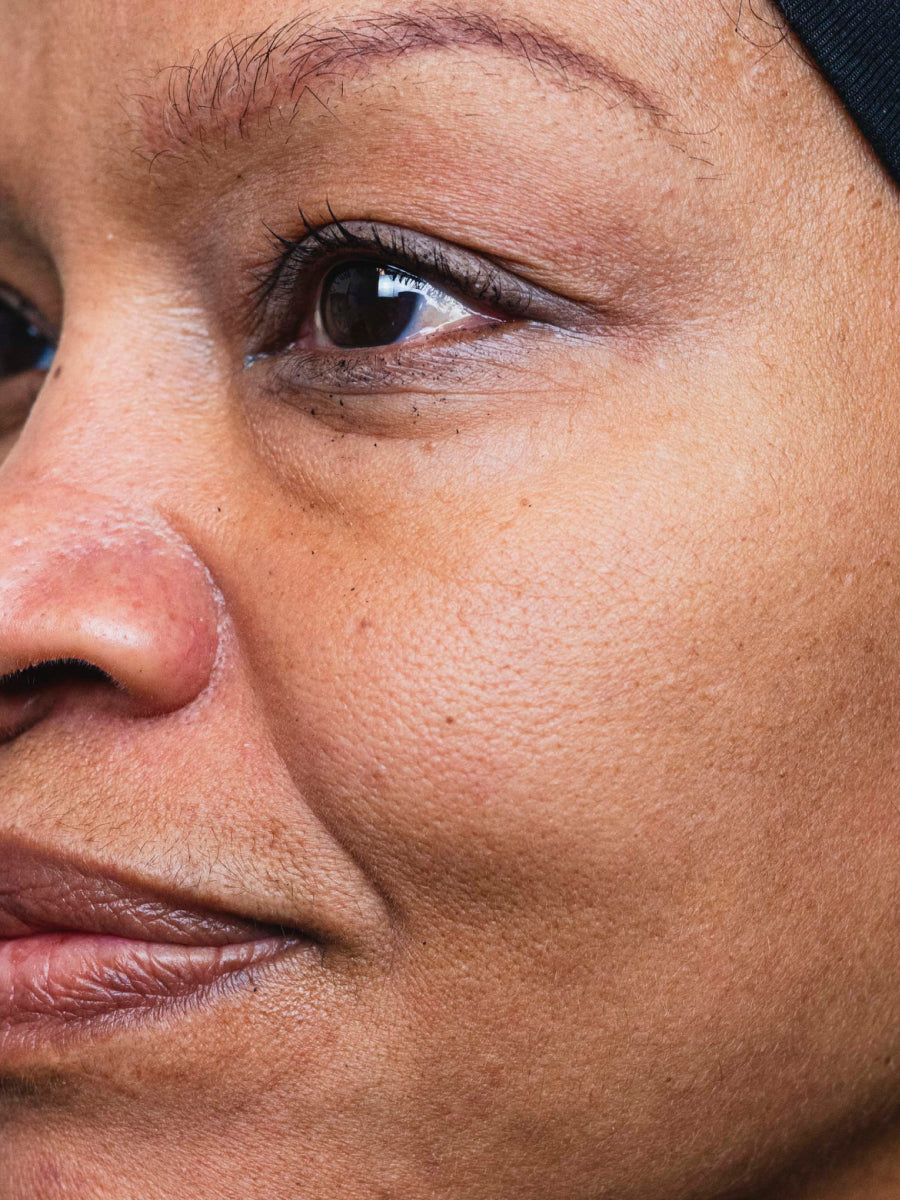

- Age: 59
- Fitzpatrick Scale: 4
- Treatment Duration: 4 weeks
- Featured Products: Circadian Cream
Featured in this trial

Circadian Cream
Overnight Collagen Seal
$135


- Age: 40
- Fitzpatrick Scale: 4
- Treatment Duration: 8 weeks
- Featured Products: Circadian Cream
Featured in this trial

Circadian Cream
Overnight Collagen Seal
$135
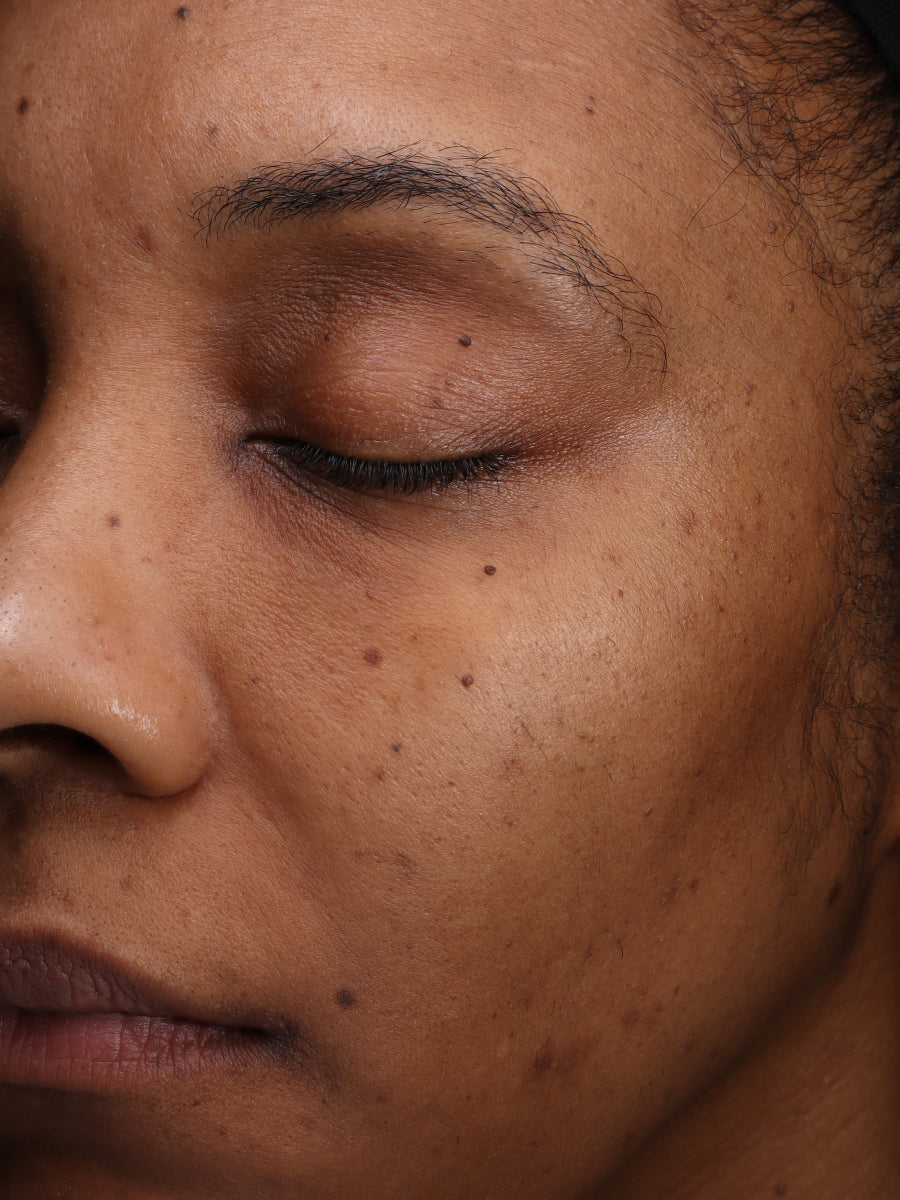
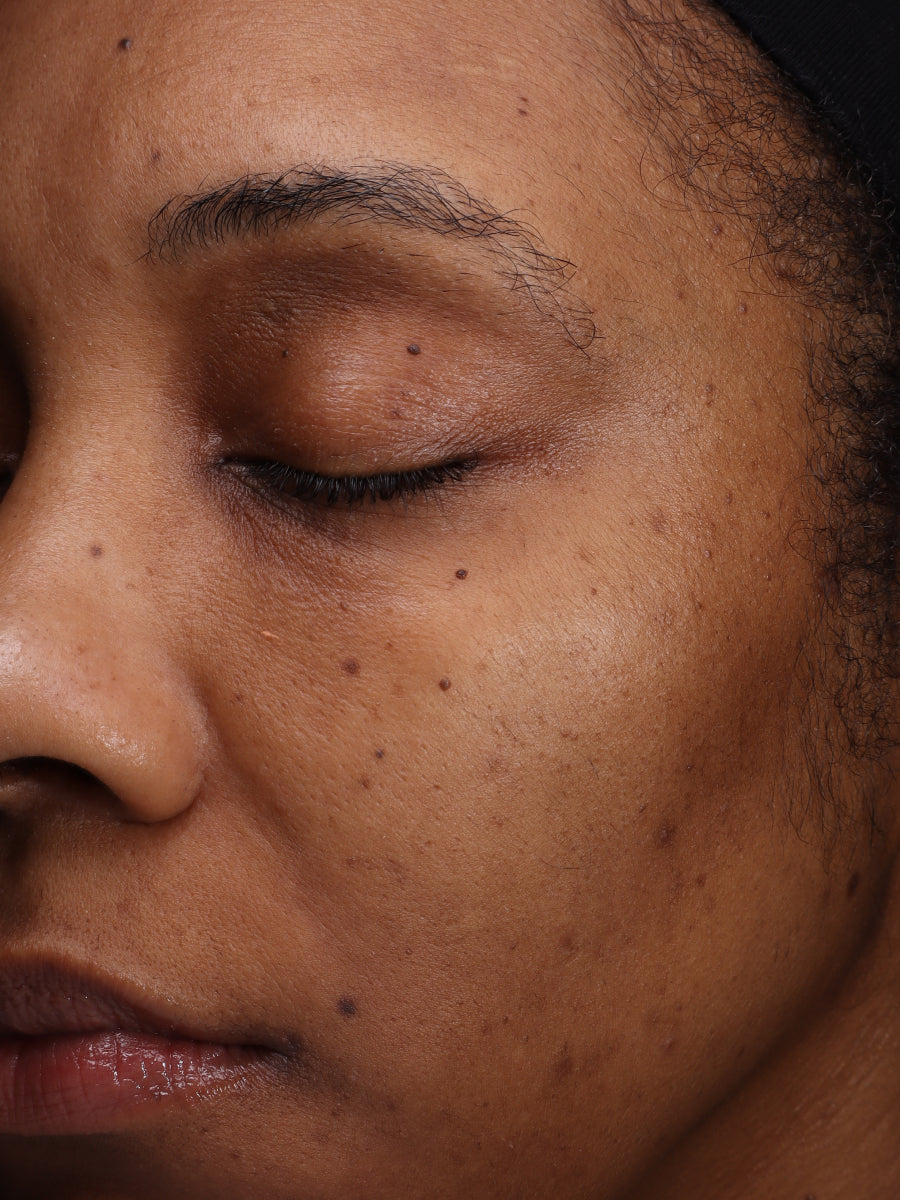
- Age: 30
- Fitzpatrick Scale: 4
- Treatment Duration: 8 weeks
- Featured Products: Pigment Processor
Featured in this trial

Pigment Processor
Daily Brightening Serum
$115


- Age: 27
- Fitzpatrick Scale: 5
- Treatment Duration: 4 weeks
- Featured Products: Pigment Processor
Featured in this trial

Pigment Processor
Daily Brightening Serum
$115